Vision for 2040
What is a Carbon Management Business Park?
Explore the carbon management and clean energy industries that could be a part of Kern County’s clean energy future.

INDUSTRY
Introduction
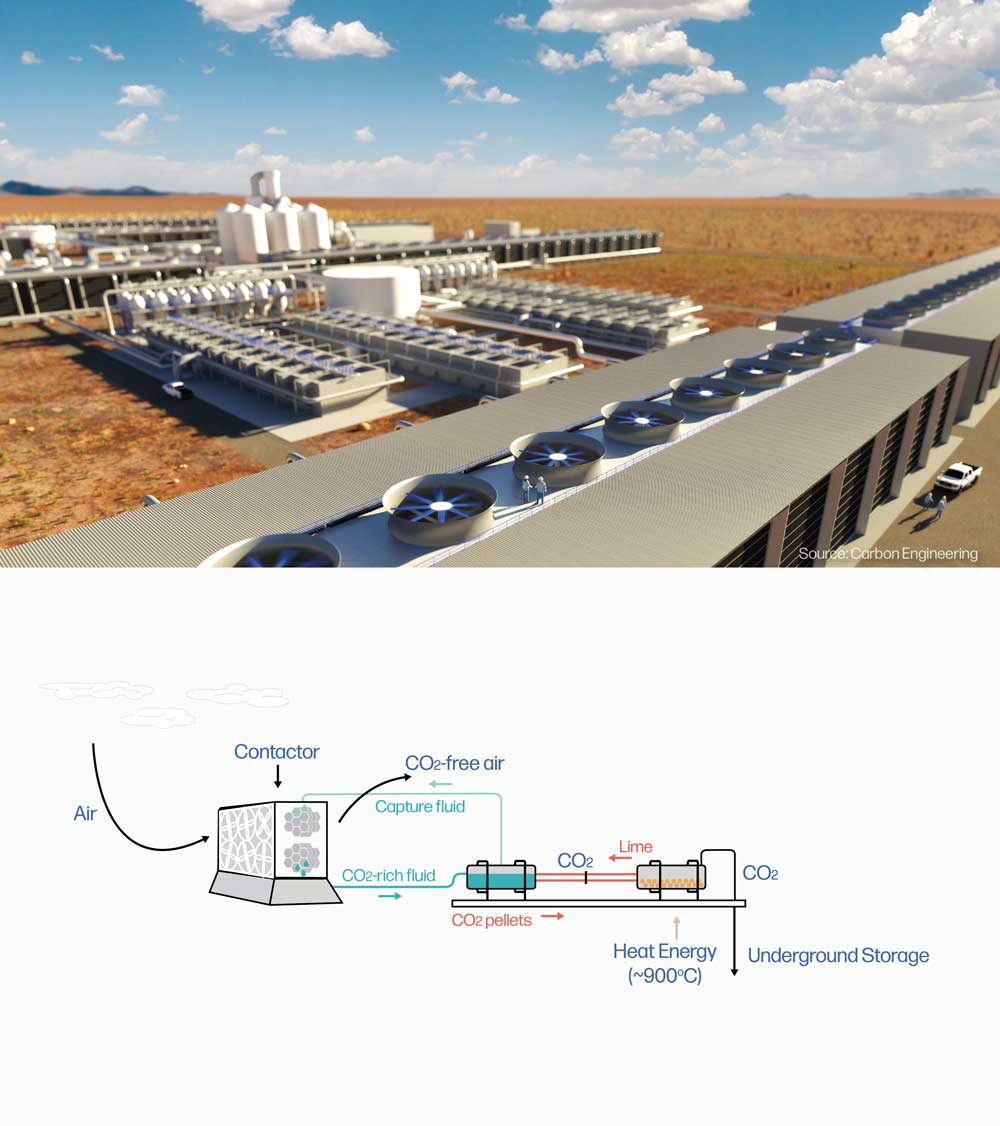
L-DAC
Direct Air Capture (DAC) is a technology that captures carbon dioxide (CO2) directly from the atmosphere. Fans or wind drive ambient air through a contactor unit, where a chemical sorbent selectively traps CO2 but allows the other components of the air to pass through and exit the system.
Liquid direct air capture (L-DAC) uses a liquid solvent, usually a hydroxide solution, as the chemical sorbent material. After CO2 is trapped in the liquid solvent, it is reacted with lime to form small carbonate pellets. The liquid solvent can be re-used to trap more CO2, and the pellets are heated to 800-1000°C, breaking down to their constituent parts: lime and a pure stream of CO2. This step is called regeneration. The CO2 is ready to be pressurized and transported for permanent storage, and the lime can be reused to pelletize more CO2.
Technological
Industry Development
- Stage of development: early
-
Existing facilities globally: 1
(Canada, captures 365 metric tons/yr) -
Facilities in development globally: 1
(Texas, will capture 1 million metric tons/yr, operational in 2024)
Energy Requirement
If energy were supplied entirely using solar power, it would take ~7,000 acres (reported range is 4,400-9,000 acres) to capture 1 million metric tons of CO2. However, solar PV-provided energy cannot directly supply heat at the temperatures needed for regeneration. Thus, L-DAC requires a high-temperature clean energy heat-source, such as an industrial heat battery or green hydrogen (H2) fuel. If H2 fuel supplied the heat energy for L-DAC, 1,400 acres of solar fields could supply the electricity demand.
- L-DAC requires ~2.8 MWh of energy for every metric ton of CO2 captured (estimates range from 1.8-3.7 MWh per metric ton CO2)
- 80-100% of that energy is for heating. 0-20% is for electricity.
Footprint
- Each L-DAC contactor unit captures ~300-600 metric tons per year, and units are modular and stackable. Thus, footprints vary depending on how high units are stacked or how they are spread out.
- To capture 1 million metric tons of CO2 per year, we estimate a facility would require about 200 acres of space. Reported estimates range from 50 to 1730 acres, depending on how contactor units are arranged.
- L-DAC units can be sited anywhere, as the only feedstock is ambient air.
An L-DAC facility capturing 1 million metric tons of CO2 annually requires
~200 acres of space
and ~7,000 acres of solar energy + industrial heat battery storage
The energy needed to power an L-DAC facility capturing 1 million metric tons of CO2 annually could power ~500,000 homes
Societal
Job Growth Potential
An L-DAC facility capturing 1 million metric tons CO2 annually could produce about 75-270 permanent jobs in operation and maintenance, requiring skills that are transferable from other industrial repair and maintenance work industries.
Such a facility would also produce about 700-1,000 construction + installation jobs, as well as thousands of indirect jobs, such as those needed to construct solar fields to support the facility.
Location Equity
Noise levels = 50-70 decibels per contactor unit - that’s about as loud as a dishwasher or vacuum. A 1 million metric ton capture facility would need ~1,600 contactor units, spread over ~200 acres.
Impact will depend on how noise scales with additional contactor units (warrants further investigation) and the distance from urbanized areas.
Depending on site location, additional jobs could increase local traffic and employees could have long distance commutes.
An L-DAC facility capturing 1 million metric tons of CO2 could produce
75-270 permanent jobs in operation and maintenance
~700-1,000 construction jobs
+
thousands of indirect jobs
Environmental
Water Requirements
L-DAC requires significant amounts of water to dilute the solvent solution used to capture the CO2, but that water + solvent solution is continuously recycled through the system, so water only needs to be replenished to compensate for evaporation. Average temperatures and humidity levels in Kern County would cause about 5 metric tons of water consumption per ton of captured CO2 in the winter, and about 15 tons of water consumed per ton CO2 in the summer. Very hot days with humidity levels below 20% could cause greater than 20 metric tons of water consumption per ton CO2 captured.
Emissions, Byproducts & Waste
L-DAC facilities are expected to produce zero or near-zero emissions onsite that could be hazardous to the environment or human health.
Neither wastewater nor hazardous waste is generated in significant amounts in L-DAC facilities.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
An L-DAC facility capturing 1 million metric tons of CO2 would use up to
7,500 acre-feet of water each year
and produces near-zero waste or emissions
Economic
Cost of Operation
To compare costs across carbon management industries, a ‘Lifetime Cost Assessment’ (LCA) model is used, which is the total cost per metric ton CO2 resulting from the cost of building the facility (capital costs), the cost of maintenance and labor (operational costs) and the cost of energy (heat + electricity), over the lifetime of the plant. For an average L-DAC facility capturing 1 million tons CO2 per year:
- Cost to build would be ~$470 million to $1.3 billion. If it ran for ~30 years, the capital costs over the lifetime of the facility would be ~$160-300 per ton of CO2.
- Annual costs for operation staff and maintenance range from ~$60-110 per ton CO2.
- Energy costs range from ~$45-257 per ton CO2, with the variation reflecting different energy sources and uncertainty in energy requirements. These costs will also vary over time due to fluctuations in energy markets.
Potential Sources of Revenue
There are two potential sources of revenue for direct air capture:
-
Federal + State incentives:
- Federal Incentive 45Q provides a tax benefit for CO2 capture and storage using DAC of $180 per ton stored.
- California's Low Carbon Fuel Standard (LCFS): credits technologies that reduce transportation-derived greenhouse gas emissions. (DAC qualifies because it removes CO2 produced by gas-powered cars and trucks.) From 2018-2022, the LCFS credit has ranged from $62-218.
- Private markets: Private businesses have purchased carbon storage at rates in the $600-1,000 per ton of CO2 range for the most environmentally-secure types of storage.
An L-DAC facility capturing 1 million metric tons of CO2 built today would cost
$265-667 per ton CO2
Federal + State incentives (~$242-398/ton CO2)
Private carbon credit market (> $600/ton CO2)
Introduction
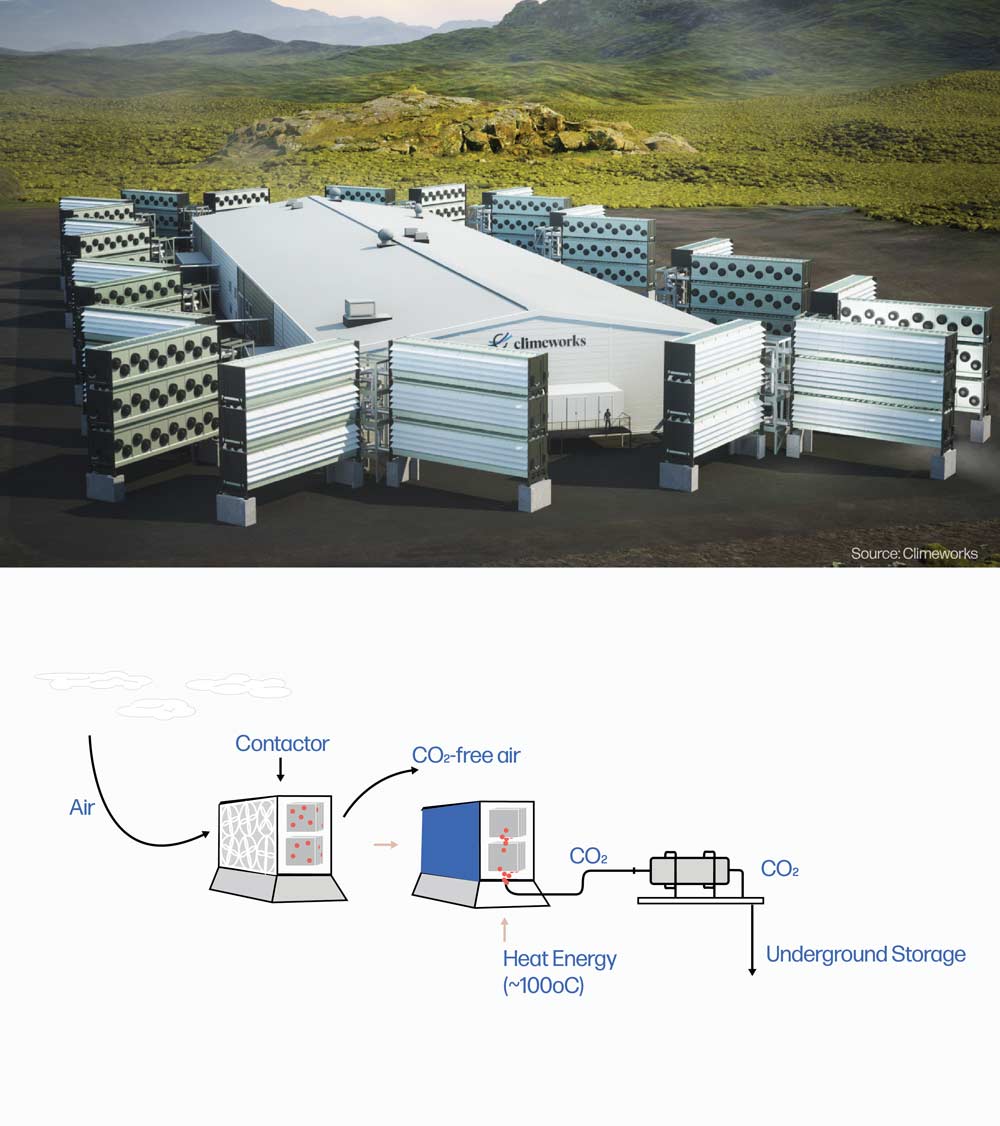
S-DAC
Direct Air Capture (DAC) is a technology that captures carbon dioxide (CO2) directly from the atmosphere. Fans or wind drive ambient air through a contactor unit, where a chemical sorbent selectively traps CO2 but allows the other components of the air to pass through and exit the system.
Solid direct air capture (S-DAC) uses a solid chemical sorbent, usually an amine-based compound, to capture atmospheric CO2. The sorbent works like a sponge, with CO2 binding to the surfaces of the solid sorbent material, as air passes across it. When the surfaces are saturated with CO2, the contactor fans and exhaust openings are closed and the unit is heated to ~100°C (212°F), causing the CO2 to release from the sorbent material. The CO2 can be removed from the unit with vacuums, now ready for transport and permanent storage. When the contactor unit is cooled and reopened, the capture process begins again, reusing the same sorbent material.
Technological
Industry Development
- Stage of development: early
- Existing facilities globally: 17 pilot + commercial (captures 6,900 metric tons/yr, combined)
- Facilities in development globally: 1 (Iceland, will capture 36,000 metric tons/yr)
Energy Requirement
S-DAC requires ~1.5 MWh of energy for every metric ton of CO2 captured (estimates range from 1.1-2.0 MWh per metric ton CO2). 75-80% of that energy is for heating. 20-25% is for electricity.
If energy were supplied entirely using solar power, it would take ~3,600 acres (reported range is 2,675-4,900 acres) to capture 1 million metric tons of CO2. Unlike L-DAC, where the heat requirements exceed what renewable resources like solar and wind can supply directly, S-DAC can be run entirely by renewable energy, even to meet the heat requirements to break the bonds between the sorbent and CO2. However, to run an S-DAC facility continuously, battery storage of solar or wind energy would also be necessary.
Footprint
Each S-DAC contactor unit captures ~500 metric tons per year. These units are modular, stackable, and about the size of a shipping container. The footprint of the site will depend on how high the contactors are stacked or how far apart they are placed from one another. To capture 1 million metric tons of CO2 with S-DAC contactors, we estimate a footprint of approximately 235 acres, but reported estimates range from 50 to 495 acres. These contactors can theoretically be placed anywhere, as the only required feedstock on-site is ambient air.
An S-DAC facility capturing 1 million metric tons of CO2 annually requires
~235 acres of space
and ~3,600 acres of solar energy + battery storage
The energy needed to power an S-DAC facility capturing 1 million metric tons of CO2 annually could power ~135,000 homes
Societal
Job Growth Potential
An S-DAC facility capturing 1 million metric tons CO2 annually could produce about 100-270 permanent jobs in operation and maintenance, requiring skills that are transferable from other industrial repair and maintenance work industries.
A new S-DAC facility would also generate approximately 700 construction and installation jobs in the region, as well as indirect jobs, like solar installation jobs to help support the facility’s energy needs.
Location Equity
Noise levels = 50-70 decibels per contactor unit - that’s about as loud as a dishwasher or vacuum. A 1 million metric ton capture facility would need ~2,000 contactor units, spread over ~235 acres.
Impact will depend on how noise scales with additional contactor units (warrants further investigation) and the distance from urbanized areas
Depending on where an S-DAC facility was placed, additional jobs could increase local traffic and employees could have long commutes.
An S-DAC facility capturing 1 million metric tons of CO2 could produce
100-270 permanent jobs in operation and maintenance
~700 construction jobs + thousands of indirect jobs
Environmental
Water Requirements
The solid sorbent material used to capture CO2 in S-DAC facilities also binds with water vapor from the atmosphere. The more humid conditions are, the more water vapor condenses onto the sorbent material along with CO2. When the contactor unit is closed and heated to release the CO2 from the sorbent, the water will be heated back into vapor and removed along with the CO2 in a gaseous stream. Then, the CO2 and water can be separated from one another, and the water could be used by local communities or other facilities in the park.
Emissions, Byproducts & Waste
DAC facilities are expected to produce zero or near-zero emissions onsite that could be hazardous to the environment or human health.
There should be no significant amount of hazardous materials generated in S-DAC facilities.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
An S-DAC facility capturing 1 million metric tons of CO2 could capture between
650 and 1,650 acre-feet of water each year for use
and produces near-zero waste or emissions
Economic
Cost of Operation
To compare costs across carbon management industries, a ‘Lifetime Cost Assessment’ (LCA) model is used. The LCA is the total cost per metric ton CO2 resulting from the cost of building the facility (capital costs), the cost of maintenance and labor (operational costs), and the cost of energy (heat + electricity), over the lifetime of the plant. For an average S-DAC facility capturing 1 million tons CO2 per year:
- Cost to build would be ~$630 million to $1.7 billion. If it ran for ~30 years, the capital costs over the lifetime of the facility would be ~$76-205 per ton of CO2.
- Annual costs for operation staff and maintenance range from ~$11-29 per ton CO2.
- Energy costs range from ~$0-278 per ton CO2, with the variation reflecting different energy sources and uncertainty in energy requirements. These costs will also vary over time due to fluctuations in energy markets.
Potential Sources of Revenue
There are two potential sources of revenue for direct air capture:
-
Federal + State incentives:
- Federal Incentive 45Q provides a tax benefit for CO2 capture and storage using DAC of $180 per ton stored.
- California's Low Carbon Fuel Standard (LCFS): credits technologies that reduce transportation-derived greenhouse gas emissions. (DAC qualifies because it removes CO2 produced by gas-powered cars and trucks.) From 2018-2022, the LCFS credit has ranged from $62-218.
- Private markets: Private businesses have purchased carbon storage at rates in the $600-1,000 per ton of CO2 range for the most environmentally-secure types of storage.
An S-DAC facility capturing 1 million metric tons of CO2 built today would cost
$87-512 per ton CO2
Federal + State incentives
(~$242-398/ton CO2)
Private carbon credit market
(> $600/ton CO2)
Introduction

Steel Micro Mill
Point source carbon capture is a technology that captures carbon directly from the exhaust stream of an industrial or power facility. Several industries are well suited for point source capture (petrochemical processing, fertilizer production, steel and cement production, and power generation), and there are in fact 34 operational facilities utilizing point source capture in the world today. Of these industries, iron and steel production is being explored as a potential industry in Kern County, with a proposed micro mill in the planning and permitting phase of development. Here, we assess the potential of integrating a steel mill into a carbon management park.
There are two main types of steel mills. Integrated mills are large (producing 3 million tons or more of steel annually) and produce steel directly from iron ore by reducing it to metal iron in the presence of coke (a coal derivative), and then refining it further to achieve a steel with optimal properties. Both steps are extremely energy intensive, and emit collectively about 1.5-2.4 tons of CO2 per ton of steel produced. However, these facilities are typically built close to sources of metallurgical coal (to make the coke) and iron ore, and thus would not be a good fit for Kern County.
The other type of steel mill is the mini or even smaller micro mill. These mills primarily melt steel scrap into new steel products using an electric arc furnace, which is much less energy intensive than the basic oxygen furnaces of integrated mills. When steel scrap is scarce, micro mills can be equipped to produce steel directly from iron ore using direct iron reduction, also a less energy and CO2 emissions intensive process than integrated mills. Mini and micro mills typically produce less than a million tons of steel annually, and their emissions intensity depends on their design.
A scrap-only micro mill powered exclusively by renewable energy does not create appreciable CO2 emissions. Alternatively, micro mills could be powered by oxyfuel combustion of natural gas, renewable natural gas, or syngas - a process in which gas is combusted in the presence of pure oxygen in a controlled environment, producing only pure streams of CO2, water, and electricity. Finally, if direct reduced iron (DRI) is produced within the micro mill, carbon can be captured from the flue gas of the processing facility.
We considered three micro mill scenarios: a scrap-based micro mill powered by solar energy (~0 tons CO2 emitted per ton of steel), a scrap based micro mill that utilizes oxy-fuel combustion (~0.15 tons CO2 emitted per ton of steel), and a micro mill with DRI that utilizes point source capture and oxyfuel combustion (~1.3 tons CO2 emitted per ton of steel). For full emissions ranges of each scenario, refer to Section 5 of the detailed report.
Technological
Industry Development
- State of development for low- or no-carbon steel: early commercial
- Existing facilities globally: 1 (DRI with carbon capture, in United Arab Emirates)
- Low- or no-carbon steel facilities in development globally: 3 (Colorado, Massachusetts, Sweden)
Energy Requirement
The primary energy needs for a scrap-based steel mill come from the electric arc furnace (EAF), which requires about 0.4-0.9 MWh of energy per ton of steel produced. Casting, rolling and finishing the hot steel that is made in the EAF into usable products requires another ~0.01-0.6 MWh/ton steel. If DRI is made on site from pelletized iron ore feedstock, it would contribute an additional 3.0-4.5 MWh of energy needs, increasing the facility’s energy requirements by an order of magnitude. DRI requires temperatures of 900-1000°C to reduce the iron ore in the presence of a reduction agent like natural gas, syngas or hydrogen. Such high temperatures cannot be directly supplied by solar-derived electricity without also utilizing a heat battery (see our section on energy storage).
A 770,000 ton steel micro mill utilizing only scrap and solar energy would need about 690-2,900 acres of solar fields to supply the electricity demand. A 770,000 ton DRI micro mill using solar energy and only capturing CO2 from process emissions of iron reduction would need 6,900-11,900 acres of solar fields (with heat battery storage) to operate. However, in this scenario, only 320,000 tons of CO2 could be captured annually. A micro mill using oxy-fuel combustion with carbon capture as the sole power supply would require no solar input.
Footprint
A steel mill that utilized DRI and oxy-fuel combustion (the most emissions-intensive scenario) would need to produce about 770,000 tons of steel annually to emit 1 million tons of CO2. The same size facility utilizing only scrap and solar energy, or scrap and oxy-fuel combustion, would emit near 0 tons CO2 and ~115,000 tons CO2, respectively. Based on the average facility footprint of six steel mini and micro mills built or in development in the United States within the last 5 years, a 770,000 ton steel micro mill would require about 510 acres of land.
A 770,000 ton steel micro mill could capture 0-1 million metric tons of CO2 annually, and requires
~510 acres of space
and 0-11,255 acres of solar energy + battery storage
The energy needed to power a 770,000 ton steel micro mill could power ~100,000-420,000 homes
Societal
Job Growth Potential
On average, it takes 0.5-1.5 man-hours to produce a ton of steel, depending on mill efficiency and automation. Given this range, a 770,000 ton steel mill could produce about 200-600 full-time equivalent jobs, a range consistent with estimates from recently built U.S. micro mills (which, scaled to 770,000 ton steel production capacity, would produce on average ~400 permanent jobs). Reported annual salaries for steel micro mill manufacturing jobs range from $60,000-$140,000 plus benefits, with higher salaries associated with technical positions. 400-600 thousand ton steel mini mills that are currently being developed in Arkansas and North Carolina report generating 500-600 construction jobs, which scaled to a 770,000 ton facility could provide up to 900 short term jobs in construction. Additionally, such a facility would produce thousands of indirect jobs to support facility needs, such as solar and battery installation jobs.
Location Equity
Steel mills do emit fine materials and gases that can have harmful health effects, like particulate matter, lead, nitrous oxides (NO2), and sulfur oxides (SOx), although there are techniques mills can use to trap matter and minimize emissions. Any mill developed within the carbon management park would be required to submit an environmental impact report and undergo pre-development review and operational monitoring to ensure emissions impacts remain within federal and state determined safety standards.
Steel mill operations are also loud - with most operational equipment emitting noise at levels that require hearing protection for workers. It is possible to design steel mills in ways that diffuse noise levels to minimize noise pollution in the vicinity. One steel mill in New Zealand integrated several noise-minimizing solutions into their facility, and measured noise levels about 0.75 miles from the facility of ~45 decibels, a typical sound level for an urban neighborhood.
A 770,000 ton steel micro mill could capture 0-1 million metric tons of CO2 annually, and could produce about
200-600 permanent jobs in technical positions, operations and administration
~700-900 construction jobs
+ thousands of indirect jobs
Environmental
Water Requirements
Steel mills require large volumes of water to operate, primarily for cooling, but secondarily for equipment descaling, dust scrubbing and other processes. About 90% of the water can be treated and reused or returned to the source, and water that is used for cooling never actually comes into contact with material or equipment. Seawater has been used for this purpose – it is possible that gray (reclaimed) water could be used similarly, minimizing the impact on local fresh water sources. If water is recirculated within the steel mill to minimize waste, it needs to be cooled and desalinated between uses. If a thermal network were integrated into a carbon management park, waste heat in the form of hot process water from the steel mill could potentially supply heat energy to other co-located industries. On average, steel mills that use electric arc furnaces require a water intake of 7,400 gallons of water per metric ton of steel produced, but discharge 7,000 gallons of water, for a net water requirement of about 400 gallons per metric ton of steel.
Emissions, Byproducts & Waste
In the electric arc furnace (the steel making phase of production), scrap metal is “charged”, or combined with DRI, pig iron (from integrated mills) and/or limestone, to add or remove impurities in the steel that control its properties (like strength and ductility). Elements that are separated out of the steel form a metal-oxide rich slag and small amounts of CO2 (not enough to be economically captured) and other gas or fine particulate emissions. The slag is a waste byproduct that can typically be used in other industrial processes, and therefore poses little environmental risk.
Particulate emissions emitted from the EAF (and to a lesser degree from other steps in the steel making process) include particulate matter composed of iron and iron oxides, and depending on the composition of the source iron, could also include heavy metals such as zinc, chromium, nickel, lead and cadmium. Gaseous pollutants such as NOx, CO and SO2 can also be emitted. These emissions can be captured and scrubbed from facility exhaust streams, to keep facility emissions under regulated limits. However, regular maintenance and monitoring of facilities is critical to prevent fugitive emissions.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
A 770,000 ton steel micro mill could capture 0-1 million metric tons of CO2 annually, and would use about
1,000 acre-feet of water each year
and produce particulate and gas emissions, waste water that can be reused, and solid waste in the form of slag.
Economic
Cost of Operation
To compare costs across carbon management industries, a ‘Lifetime Cost Assessment’ (LCA) model is used. The LCA is the total cost per metric ton CO2 resulting from the cost of building the facility (capital costs), the cost of maintenance and labor (operational costs), and the cost of energy (heat + electricity), over the lifetime of the plant. However, for a micro steel mill, the cost of carbon capture is a comparatively small addition to the cost of building the mill. For simplicity, we assess the costs of creating the mill and of capturing carbon from the mill separately. A micro steel mill that uses scrap and clean energy (0 tons CO2 emissions per ton steel) would cost about $398-848 per ton steel produced. A scrap mill that used oxy-fuel combustion for energy would cost ~$407-903 per ton steel produced, with the added cost of CO2 capture being ~$68-112 per ton CO2. A mill that used direct reduced iron (DRI) with point source carbon capture and oxy-fuel combustion would cost ~$564-946 per ton steel produced, and the cost of CO2 capture would be $38-112 per ton CO2.
For a 770,000 ton steel micro mill that could capture up to 1 million tons CO2 per year:
- Cost to build would be ~$460 million to $1090 million. Over the lifetime of the steel mill, the capital costs would be ~$88-125 per ton of steel produced.
- Fixed operational costs (including labor, maintenance, and other fixed expenses) would range from ~$77-116 per ton of steel produced. Raw material costs depend on whether iron is sourced primarily from scrap ($192-557 per ton scrap) or iron pellets for producing DRI ($81-154 per ton iron).
- Energy costs also depend on the material used. Solar energy costs for a scrap-EAF mill are $41-50/ton steel, natural gas costs for a scrap-EAF mill are $40-88/ton steel, and natural gas costs for a DRI-EAF mill are $211-461/ton steel.
- The addition of carbon capture via oxy-fuel combustion on a scrap-EAF mill powered by natural gas (or syngas or RNG) would be $10-17/ton steel. The addition of both oxy-fuel carbon capture and point source carbon capture on a DRI-EAF facility would cost $75-118/ton steel.
Potential Sources of Revenue
There are 3 potential sources of revenue for steel production:
- Selling steel: The current price of steel in the U.S. is $1249-1719 per ton, depending on the type of finished steel product, although this price has increased significantly since 2020 due to global inflation and supply chain issues related to the Covid-19 pandemic.
- Federal + State incentives: Federal Incentive 45Q provides a tax benefit for CO2 capture and storage processes other than direct air capture (DAC) of $85 per ton stored. Another possibility, explored in more depth in the downloadable report, is replacing natural gas with hydrogen as the mill fuel and iron reducing agent. A federal tax credit for near-zero emissions hydrogen production could make green hydrogen-steel technology cost competitive with other approaches, although this technique is still in early stages of development. Finally, both the federal and California governments have enacted ‘Buy Clean’ incentives, requiring federal and state building contracts to buy low-carbon building materials, thus increasing the competitiveness of these more expensive steel products.
- Private markets: Private businesses have purchased carbon storage at rates in the $600-1,000 per ton of CO2 range for the most environmentally-secure types of storage.
Of the two types of carbon credit incentives, facilities must choose between the federal + state incentives and private markets - the same ton of carbon captured is not eligible for both revenue streams.
A steel micro mill capturing up to 1 million metric tons of CO2 would cost about
$38-112 per ton CO2.
Federal + State incentives
(~$85/ton CO2)
Private carbon credit market
(> $600/ton CO2)
Introduction



R&D Incubator
A facility of the scale envisioned for this business park would make Kern County a global leader in the emerging carbon management industrial sector. By creating a space within the park where new ideas and relationships between industry, researchers and start ups are cultivated,
Kern County would not only lead in development of carbon capture capacity, but also in driving innovation in new carbon management technologies.
A Research & Development Incubator, sited within the industrial park, is envisioned as a fully permitted space for newcomers in carbon management industries to innovate, to scale from the lab to a pilot project, and to showcase “proof of concept” prototypes that are ready for further development. It would allow such newcomers to access mentorship and technical guidance from a consortium of industry partners, community organizations, and regional academic institutions and national labs, including California State University, Bakersfield, Kern County Community College District, University of California, Merced, and Lawrence Livermore National Lab. For local universities, the incubator would provide facilities for workforce development, fieldwork opportunities for students and faculty, and a nexus for academic-industry research collaborations.
Introduction

BiCRS
Biomass Carbon Removal and Storage (BiCRS) takes carbon captured by plants through photosynthesis and prevents it from re-entering the atmosphere by storing it underground. Often, useful byproducts like fuels, electricity, and soil amendments, are created in the process. BiCRS projects aim to maximize the amount of carbon captured from organic materials, while their counterpart – Bioenergy with Carbon Capture and Storage (BECCS) – uses similar processes while trying to maximize the energy output from biomass.
There are multiple processes available to process biomass into energy + carbon byproducts, a campfire being the most simple and longest used. From an industrial perspective, bioenergy production ranges from well-understood biochemical processes – like turning corn into ethanol – to new, innovative high-heat thermochemical processes like pyrolysis and gasification. Pyrolysis and gasification use heat, pressure and other materials to break down organic matter. In pyrolysis, biomass is heated to temperatures between 500-700°C (~930-1300°F) in a chamber without oxygen to produce a solid called biochar, a liquid bio-oil, and a “syngas” made up of CO2, H2, CO, and light hydrocarbons. The speed and temperature of this process determines the ratio of solid, liquid, and gaseous products. In gasification, biomass is heated to temperatures over 700°C (~1300°F) with oxygen under controlled conditions, causing the organic material to break down into gaseous CO, CO2, and H2 rather than combusting. In each of these processes, CO2 (and CO converted to CO2) can be separated from the other byproducts for transport and underground storage.
Technological
Industry Development
- Stage of development: early commercial
- Existing facilities globally: 16
- Facilities in development globally: 50
Energy Requirement
The energy needed to power a BiCRS/BECCS facility capturing 1 million metric tons of CO2 annually is typically less than the total energy output, meaning that as facilities generate energy in the form of electricity or synthetic fuels for consumers, they can use part of the energy they generate to power their own operations.
BiCRS/BECCS is capable of generating about 0.83 MWh of energy for every metric ton of CO2 captured. Estimates are approximate, as many bioenergy projects only report their energy output, not the total energy generated or the amount used to sustain their pyrolysis or gasification reactions.
Due to the high temperatures needed for pyrolysis or gasification, facilities generally require an energy source for heat. For BiCRS/BECCS, this energy is typically supplied by the biomass itself, which contains energy, or by the fuels (syngas, hydrogen, or other products) that are produced on-site by breaking down biomass. Depending on the model, some pyrolyzers need external fuel to start the process, which can be supplied from renewable sources like green hydrogen or renewable natural gas (RNG).
Footprint
A BiCRS/BECCS facility footprint is often relatively contained, with 1-3 large buildings (three or more stories in height, to accommodate the reactor equipment), space to store biomass before processing (this can be indoors, outdoors, or a mix), and space to hold any byproducts that need to be disposed of or sold. The tallest feature is typically a chimney stack or gas flare - these can be as much as 100 feet tall. On-site footprint estimates for BiCRS/BECCS facilities have been declining over the past decade, and pyrolysis and gasification facilities are notably smaller than bioenergy plants.
The primary land use requirements for a BiCRS/BECCS facility are dominated by the land required to generate biomass. Since most facilities are hoping to use waste biomass, leftover from agricultural or forestry operations, this should not contribute to the on-site land footprint.
A BiCRS/BECCS facility capturing 1 million metric tons of CO2 annually requires
180-400 acres of space
and low to no external energy input
The energy produced by a BiCRS/BECCS facility capturing 1 million metric tons of CO2 annually could power ~75,000 homes.
Societal
Job Growth Potential
A BiCRS/BECCS facility capturing 1 million metric tons CO2 annually could produce about 45-150 permanent jobs in operation and maintenance, primarily requiring skills that are transferable from other industrial repair and maintenance work industries. Estimates may vary depending on the specific processes at a facility.
A new BiCRS/BECCS facility would also generate anywhere from 1,000 to 4,000 construction and installation jobs in the region, as well as indirect jobs, like solar installation jobs to help support the facility’s energy needs.
Location Equity
Noise levels of BiCRS/BECCS processing facilities are relatively low, because the equipment used for pyrolyzers and gasifiers are housed inside buildings that can reduce noise distribution. Outdoor equipment that may cause noise includes moving conveyor belts and fans, which are not louder than typical farm equipment. Careful site planning can further reduce noise distribution from the equipment.
A BiCRS/BECCS facility requires significant daily feedstock additions - if biomass is not supplied by rail, it would increase local traffic. CO2 output to biomass feedstock ratios are about 0.9-1.5, meaning that a facility capturing 1 million tons of CO2 annually would need about 1,800-3,000 tons of feedstock delivered daily. That’s about 90-150 tractor-trailer truckloads each day. Additionally, depending on where the facility is sited, employees could have long commutes.
A BiCRS/BECCS facility capturing 1 million metric tons of CO2 could produce
45-150 permanent jobs in operation and maintenance
1,000-4,000 construction jobs
+ thousands of indirect jobs
Environmental
Water Requirements
Due to the range of biomass types, temperature, pressure, and other factors impacting how biomass breaks down in a BiCRS/BECCS facility, water use can vary dramatically. For processes that are upgrading gaseous products into synthetic fuels or hydrogen fuel, water use typically rises to help purify the gas stream and generate desirable alternative fuels. For some other processes, facilities have found they can actually produce excess water that can be used directly for irrigation or other purposes. An example is oxy-fuel combustion - a process that combusts syngas derived from pyrolysis (or other forms of methane) in a controlled environment in the presence of oxygen, such that the byproducts are only a pure stream of CO2 and water, as well as some electricity. Such a facility could be developed in tandem with a BiCRS/BECCS facility for the sole purpose of CO2 capture from biomass, or could be used to generate heat or electricity for co-located industries like steel or L-DAC.
Emissions, Byproducts & Waste
BiCRS/BECCS facilities are expected to produce few emissions onsite that could be hazardous to the environment or human health. BiCRS/BECCS facilities do emit particulate matter, as well as NOx, carbon monoxide (CO), and volatile organic compounds (VOCs). However, these emissions can be up to 95-99% lower than they would be if the biomass was burned instead, as is typical for clearing forestry and agricultural waste. Additionally, pyrolysis and gasification emissions are much lower than that used in bioenergy or waste-to-energy plants, where biomass is also combusted. Regardless, monitoring these emissions to mitigate pollution for local residents is very important.
There should be no significant amount of hazardous material generated in BiCRS/BECCS facilities, but any byproducts do need to be properly disposed of. Solid wastes, like spent organic material or catalysts, will need to be landfilled. Some other products can be sold: ash as a fertilizer, and biochar as a soil amendment, compost additive, or chemical catalyst.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
A BiCRS/BECCS facility capturing 1 million metric tons of CO2 could generate water or use up to
266 acre-feet of water per year
and produces a small amount of waste and emissions
Economic
Cost of Operation
To compare costs across carbon management industries, a ‘Lifetime Cost Assessment’ (LCA) model is used. The LCA is the total cost per metric ton CO2 resulting from the cost of building the facility (capital costs), the cost of maintenance and labor (operational costs), and the cost of energy (heat + electricity), over the lifetime of the plant. For an average BiCRS/BECCS facility capturing 1 million tons CO2 per year:
- Cost to build would be ~$350 million to $1.2 billion. If it ran for 30 years, the capital costs over the lifetime of the facility would be ~$30-189 per ton of CO2.
- Annual costs for operation are dominated by the cost of biomass feedstock - which ranges from $20-60. Fixed operation costs (primarily for staff and routine maintenance) are typically 2-6% of capital costs, or $1-11. Variable operation costs, which include non-biomass feedstocks, waste disposal, and unplanned maintenance, range from $10-27 per ton CO2.
- Energy costs are negligible.
Potential Sources of Revenue
There are 4 potential sources of revenue for BiCRS/BECCS:
- Selling energy: BECCS projects aim to maximize the energy output of biomass conversion, and sell a variety of products including electricity, ethanol and synthetic fuels that could be a low-carbon replacement for sectors that are hard to electrify, like shipping and aviation. A notable BiCRS product is hydrogen gas (H2), which, in addition to CO2, is generated through gasification. This can be sold for profit as a fuel or chemical feedstock, and is explored more in the Hydrogen Production - Biomass section.
- Selling byproducts: Biomass pyrolysis (and sometimes gasification) generates solid biochar, which can be sold as a soil amendment, compost additive, or chemical catalyst.
- Federal and state incentives: Federal Incentive 45Q provides a tax benefit for CO2 capture and storage of $85 per ton permanently stored for capture processes other than direct air capture (DAC). California's Low Carbon Fuel Standard (LCFS) credits technologies that reduce transportation-derived greenhouse gas emissions, and can provide credits for makers of low-carbon alternative fuels or facilities supplying low-carbon electricity for transportation users. From 2018-2022, the LCFS credit has ranged from $62-218.
- Private markets: Private businesses have purchased carbon storage at rates in the $600-1,000 per ton of CO2 range for the most environmentally-secure types of storage.
Of these two types of carbon credit incentives, facilities must choose between the federal + state incentives and private markets - the same ton of carbon captured is not eligible for both revenue streams.
A BiCRS/BECCS facility capturing 1 million metric tons of CO2 built today would cost
$61-288 per ton CO2
Federal + State incentives
(~$147-303/ton CO2)
Private carbon credit market
(> $600/ton CO2)
Introduction

Green Hydrogen from Biomass
Hydrogen production using biomass is a type of green hydrogen technology, in that it can be produced without emitting CO2 to the atmosphere (the other form of green hydrogen is electrolysis). Green hydrogen from biomass uses a multi-step process to break down organic materials into hydrogen and carbon dioxide. In the first step, gasification, organic material is heated to temperatures over 700°C (~1300°F) in the presence of oxygen and under controlled conditions, causing the organic material to break down into syngas – a mixture of CO, CO2, and H2 – rather than combusting. A second step, called a water-gas shift reaction, reacts the CO with water to form more CO2 and H2. The hydrogen can then be sold as green hydrogen, while the CO2 is captured for underground storage or use in long-lived products.
A similar reaction can convert methane (natural gas) into hydrogen, instead of biomass as the starting material, in a process known as steam methane reforming (SMR). However, even with carbon capture and storage integrated into SMR processes – a process known as blue hydrogen – only 44-88% of the carbon emissions can be captured, so the process is still net carbon-emitting.
Technological
Industry Development
- Stage of development: early
- Existing facilities globally: 0
- Facilities in development globally: 4
Energy Requirement
A biomass gasification facility producing 1 million tons of CO2 for capture can produce about 70,000 tons of hydrogen if it is powered entirely through an external source of energy. For a facility to be self-sufficient (effectively needing no external energy input), about 30% of the syngas produced in the gasification step of hydrogen production is needed. In this case, for every million tons of CO2 produced, only about 50,000 tons of hydrogen is produced. Whether and to what degree produced syngas or external energy sources are used depends on the relative cost of available energy sources, and the marketplace value of green hydrogen.
Most of the energy needs for biomass hydrogen production are used to attain the necessary heat and pressure to gasify the organic feedstock. The exact amount of required heat energy is difficult to ascertain, as the process is still relatively new. Additionally, as most facilities meet this energy need with their own supply of syngas, it is often unreported. Regardless of the scale of heat energy requirements, if solar-sourced energy were used, it would need to be paired with heat batteries, which likely would make it uneconomical compared to utilizing a portion of the syngas. As for the electricity demands, most facilities either pursue a grid connection as back-up or set up their own solar panels to meet any electrical demand. For a facility capturing 1 million metric tons of CO2, the estimated solar capacity would be 135 MW, occupying ~945 acres.
Footprint
A biomass gasification facility is typically quite small, and can be contained in only a few buildings, with an on-site gas flare and evaporation ponds to handle any waste. Footprint estimates for biomass-based facilities have been declining over the past decade. The primary land use needs are dominated by the land required to generate biomass and renewable energy to support hydrogen production. Since many facilities intend to use waste biomass, left over from agriculture or forestry operations, this should not contribute to the on-site land footprint and are not included in this estimate.
A biomass hydrogen production facility capturing 1 million metric tons of CO2 annually requires
~180 acres of space
and 0-950 acres of solar energy
+ battery storage
The energy needed to power a biomass hydrogen production facility capturing 1 million metric tons of CO2 annually could power up to ~35,500 homes
Societal
Job Growth Potential
A biomass hydrogen production facility capturing 1 million metric tons CO2 annually could produce about 45-150 permanent jobs in operation and maintenance, requiring skills that are transferable from other industrial repair and maintenance work industries.
Like other BiCRS/BECCS technologies, a new biomass hydrogen production facility would also generate approximately 1,000 to 4,000 construction and installation jobs in the region, as well as indirect jobs, like solar and battery installation jobs to help support the facility’s energy needs.
Location Equity
Much like other BiCRS facilities, noise levels for biomass hydrogen production are relatively low. The primary noise components are the conveyor belts and fans within the gasifier. The gasifier is housed within a building, dampening noise, such that outside the building ambient noise is not impacted. Designing to mitigate sound exposure to workers with absorbent materials, and requiring workers to wear ear protection on-site, can further reduce noise impacts. Any noise impact for local communities will depend on the distance from urban areas, as sound diffuses quickly with distance.
Depending on where a biomass hydrogen facility is placed, local traffic could increase due to the need to provide feedstock and new jobs, and those employees could have long commutes. Like other BiCRS facilities, biomass hydrogen production requires a significant amount of feedstock delivered daily. For a facility capturing 1 million tons of CO2 annually, about 1,800 tons of feedstock (~90 tractor-trailer truckloads) need to be delivered each day. The added traffic burden could be diminished if feedstocks were delivered by rail.
A biomass hydrogen production facility capturing 1 million metric tons of CO2 could produce
45-150 permanent jobs in operation and maintenance
~1,000-4,000 construction jobs
+ thousands of indirect jobs
Environmental
Water Requirements
Although researchers are working to reduce the water requirements needed for hydrogen production via biomass gasification, currently, water is used to perform the water-gas shift reaction to generate hydrogen and CO2 from the CO produced during the gasification step, and to purify the gaseous products. Some forms of gasification also rely on steam or supercritical water, which can increase the overall water demand. The most conservative facilities use about equal quantities of biomass and water in their process. Ongoing research efforts are aimed at using wastewater or other reclaimed water in the gasification process, to minimize the impact on freshwater resources. If used to purify gas, water is typically stored in evaporation ponds on site, and could be treated for reuse with appropriate equipment.
Emissions, Byproducts & Waste
Biomass hydrogen production facilities rely on gasification, and thus generate a small amount of various atmospheric pollutants, including particulate matter, NOx, carbon monoxide (CO), and volatile organic compounds (VOCs). The concentration of these pollutants in process emissions are as much as 95-99% lower than what is emitted through biomass combustion, which occurs during crop burning or in traditional bioenergy power plants.
There should be no significant amount of hazardous materials generated in biomass hydrogen production facilities. Evaporation ponds handle wastewater discharge, most byproducts (like ash) can be sold; less-frequently replaced items, like spent catalyst, can be landfilled or recycled by the manufacturer, depending on the material.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
A biomass hydrogen production facility capturing 1 million metric tons of CO2 would use
between 266 and 1,840 acre-feet of water each year
and produces minimal emissions, but some waste byproducts
Economic
Cost of Operation
To compare costs across carbon management industries, a ‘Lifetime Cost Assessment’ (LCA) model is used. The LCA is the total cost per metric ton CO2 resulting from the cost of building the facility (capital costs), the cost of maintenance and labor (operational costs), and the cost of energy (heat + electricity), over the lifetime of the plant. A biomass hydrogen production facility producing 1 million tons of CO2 would have a levelized cost of about $2.50-$3.60 per kilogram of hydrogen produced, which translates to an estimated range of $125-257 per metric ton of CO2 captured. With increased plant capacity, the costs of CO2 capture could decline by as much as 50%, but the capacity potential for a hydrogen biomass facility is constrained by the regionally available biomass feedstock.
For an average biomass hydrogen production facility capturing 1 million tons CO2 per year:
- Cost to build would be ~$278 million to $328 million. If it ran for 30 years, the capital costs over the lifetime of the facility would be ~$24-52 per ton of CO2.
- Operational costs would range from ~$60 million to $101 million. The feedstock is the largest amount of this cost, accounting for an estimated $44 million annually. The next big cost categories come from chemical, material, and utility costs, while labor, maintenance, and waste treatment make up a small amount of the operating expenses.
- Energy costs are variable. Self-sustaining plants—which can meet their own energy needs—are the most expensive to build, but have the lowest operational costs. Plants that receive some or all of their energy from external sources are typically cheaper to construct, but more expensive to operate and maintain.
Potential Sources of Revenue
There are 4 potential sources of revenue for biomass hydrogen production:
- Selling hydrogen: Biomass gasification generates hydrogen gas (H2), in addition to CO2. This hydrogen can be sold as a fuel or chemical feedstock. Currently, hydrogen generated from biomass gasification is not competitive with carbon-emitting forms of hydrogen production, like gray or blue hydrogen, which cost $1-2 per kg (see report for full details). However, the sale of byproducts and added financial incentives for capturing carbon can help make biomass gasification a viable low-carbon route for hydrogen production.
- Selling byproducts: Biomass gasification also generates byproducts including ash, which can be collected from the gasifier and sold as a fertilizer.
- Federal + State incentives: Federal Incentive 45Q provides a tax benefit for CO2 capture and storage processes other than direct air capture (DAC) of $85 per ton stored. A hydrogen-specific federal incentive established by the Inflation Reduction Act in 2022, Section 45V, is expected to give a tax credit of $3/kg hydrogen for a near-zero emissions hydrogen production process, but the Treasury Department is still setting the rules for eligibility. Producers can choose between the 45Q and 45V tax credits, but cannot receive both. California's Low Carbon Fuel Standard (LCFS): credits technologies that reduce transportation-derived greenhouse gas emissions. Hydrogen is an eligible alternative fuel if sold for transportation use in California. From 2018-2022, the LCFS credit has ranged from $62-218.
- Private markets: Private businesses have purchased carbon storage at rates in the $600-1,000 per ton of CO2 range for the most environmentally-secure types of storage.
Of these two types of carbon credit incentives, facilities must choose between the federal + state incentives and private markets - the same ton of carbon captured is not eligible for both revenue streams.
A biomass hydrogen production facility capturing 1 million metric tons of CO2 built today would cost about
$125-257 per ton CO2
Federal + State incentives
(~$147-303/ton CO2 or $3/kg H2)
Private carbon credit market
(> $600/ton CO2)
Agriculture
What is the technology and how does it work?
Agricultural practices can intersect with carbon management in a wide variety of ways. BiCRS and BECCS are carbon management industries that rely on biomass like crops, crop residues, or manure to fuel their operations. They can convert biomass to create electricity, hydrogen, and other biofuels.
Separate from industrial carbon management technologies, implementing sustainable farming practices like low- or no-till fields, cover crops, and monitoring sensors can make agriculture a carbon management technique on its own, sequestering carbon out of the atmosphere, while improving soil quality, and reducing fertilizer and water usage.
Why would it be co-located with carbon management technology?
Co-locating agriculture with industrial carbon management facilities can produce mutually beneficial relationships. For example, BiCRS facilities that need biomass feedstocks can source waste biomass from local farmers and can supply farms with biochar, a soil additive that can replace synthetic fertilizers. Biomethane, also known as renewable natural gas or RNG, is produced by decomposing biomass (like dairy manure), and can be used as a feedstock for some BiCRS processes, or as a fuel with carbon capture for co-located industries. There may also be potential for sharing water and land between uses.
Furthermore, carbon dioxide itself can be beneficial for some agricultural businesses. Carbon dioxide can help promote increased plant growth, so some greenhouses purchase captured carbon to apply it to their plants and increase crop yields.
What are the technical considerations?
For technology-based carbon management practices like BiCRS and BECCS, the role of agricultural partners is more logistical than technical. Farms supplying waste biomass would need to arrange details with BiCRS/BECCS facility operators like land access, pricing, and transfer of biomass waste to (and biochar from) facilities, but this should be technically non-complex.
Other carbon management tactics for farms, like sensors, no-till or low-till approaches, or cover cropping are all technologically mature avenues to reduce emissions and/or resource intensity. Details of these practices are outside the scope of this work, but more information can be found from the US Department of Agriculture.
What are the societal and environmental considerations?
With rising interest in areas like bioenergy (BECCS) and biofuels, there is increasing pressure to move from growing crops for food to growing crops, like corn or soy, to support biomass industries. Kern’s agricultural sector is a leading national source of nuts, fruits, and vegetables – switching to fuel crops would be a drastic loss for the variety and amount of these foods grown domestically. Governments and carbon management partners should ensure that biomass supplies in the region prioritize waste biomass, rather than encouraging switches to less biodiverse crops.
Additionally, using waste biomass for carbon management is a productive alternative to burning crop waste, which releases air pollutants like SO2, nitrous oxides (NOx), and particulate matter, and are a particular health hazard to the regional community. From 2000-2020, between 200,000 and 1,200,000 tons of agricultural waste were burned in the San Joaquin Valley each year. The California Air Resources Board (CARB) has mandated that agricultural burning be phased out by 2024, meaning disposal alternatives will be required for the region’s agricultural waste.
What are the economic considerations?
With potential climate change impacts like lower water availability and increased temperatures, agriculture in Kern County may need to adapt to changing circumstances. Sharing land with carbon management practices, or selling available water allotments or waste biomass supplies to these industries, could be options to augment existing agricultural income over time.


Clean Energy Industries
What is the technology and how does it work?
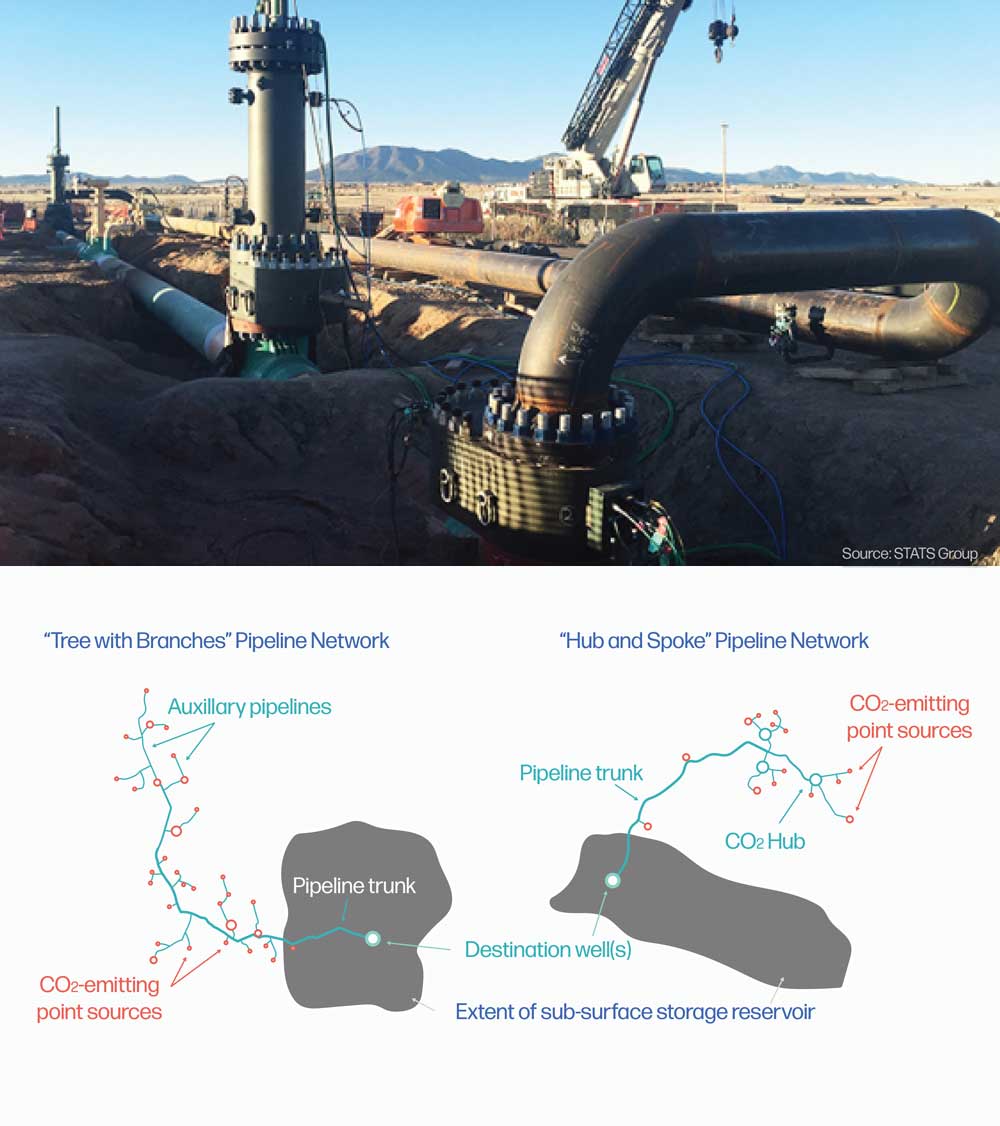
Steel pipelines are the most common transportation choice to move carbon dioxide long distances over land. CO2 can be moved through pipelines as a gas, liquid, dense phase substance, or supercritical fluid. First, the CO2 is purified, then brought to an appropriate temperature and pressure for transport. CO2 can be moved through the pipeline passively, relying on pressure and gravity, or with the assistance of pumps along the line. Pipelines often exist in a local network, either with a ‘tree with branches’ or a ‘hub and spoke’ model.
Why would it be co-located with carbon management technology?
To ensure a climate benefit from the capture of CO2, it is necessary to keep trapped carbon dioxide from returning to the atmosphere. Pipelines are typically the most efficient and cost-effective mechanism to move carbon dioxide between sources – in this case, industries capturing or generating carbon dioxide – and sinks, such as underground storage or use.
What are the technical considerations?
The footprint for a CO2 pipeline should be invisible at ground level – pipelines are typically placed about 3 feet beneath the surface.
Compared to the capture industries within a carbon management park, pipeline energy use would be minimal, especially if the route is designed to maintain appropriate pressure and utilize gravity to keep the CO2 moving through the pipeline. The largest energy input in the process would be compressing the CO2 for transport, and ranges from 90-120 kWh per metric ton of CO2 (about as much electricity as is needed to power a home for 1-2 days), depending on the required pressure and temperature conditions of the pipeline system. CO2 compression could be the responsibility of either the pipeline operator or the capturing industry.
What are the societal and environmental considerations?
Two societal and environmental considerations are of note for CO2 pipelines: job growth potential and safety. Construction of a pipeline system from a carbon management park to a storage site would temporarily generate hundreds of jobs. Monitoring and upkeep of a local pipeline network will likely create between 8-20 high-wage permanent jobs, which require a mix of skills similar to existing pipeline and other heavy industry jobs.
In regards to safety, in high concentrations, carbon dioxide is toxic to human and animal health and since it is denser than air, carbon dioxide released from a leaking pipeline will not dissipate; it will sink into low-lying areas and could impact residents. Symptoms of CO2 exposure include nausea and dizziness and, in very high concentrations, are deadly. Thus, a variety of safety measures – including seismic and computational monitoring, fracture arrestors, and placing scent or color in the gas stream for easy identification – are important to ensure continuous safe operation of a CO2 pipeline system. Additional safety measures include siting pipelines away from urban development and/or placing pipelines underground. Many of these safety measures are already common in pipeline systems that transport hazardous materials, including petroleum products, natural gas and chemicals.
The federal rulemaking agency for pipeline transport of carbon dioxide, PHMSA, is in the process of updating standards for CO2 pipelines to strengthen pipeline safety, including specific requirements related to emergency preparedness and response to any residents located nearby pipelines.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
What are the economic considerations?
Modeled costs for transporting carbon dioxide are in the range of $10-20 per ton of carbon dioxide. Pipelines with few bends in the path and that avoid heavily built areas, like cities and suburbs, are the most economically efficient to build. In general, shorter distances and greater pipeline diameters also decrease the cost per ton of carbon dioxide transport. Additionally, from a systems perspective, creating a single large diameter pipeline system from a carbon management hub, like an industrial park, rather than installing many small diameter pipelines from widely dispersed CO2-emitting facilities, is significantly more cost effective.
To develop the pipeline system, operators need to obtain rights-of-way for all segments of the pipeline path, which is most cost-effective with fewer landowners and in more rural areas. In terms of financing, pipeline operating companies would need to negotiate transport tariffs with the companies capturing and/or sequestering carbon to offset building costs and, eventually, generate revenue. Thus, for the purposes of a carbon management business park, pipelines should be considered as an added per ton cost for CO2 capture when considering the economic feasibility of each potential carbon management industry.
What is the technology and how does it work?
Battery storage is designed to store electrical or thermal energy created from intermittent sources (like wind or solar) until it is needed for use. There are many types of batteries that store energy as electricity, such as lithium-ion, metal-air, redox flow, molten salt, and compressed air storage systems. Most of these systems use various chemical elements moving back and forth across a medium to ‘charge’ and ‘discharge’ electrons, producing electricity. Thermal energy storage relies on storing energy as heat in bricks, rocks, water, or salts in an insulated environment, then releasing that heat later, often by interaction with cold water to generate steam.
Why would it be co-located with carbon management technology?
Battery storage can help carbon management facilities run continuously through the day and night, despite irregularities in the supply of renewable energy sources like solar or wind. Battery storage could also help store latent waste heat or electrical energy generated by some carbon management processes and redistribute this energy to other parts of the carbon management park, enabling energy reuse, and lowering the costs and resource demands of operations.
What are the technical considerations?
The size, complexity, and commercial readiness of different energy storage technologies vary considerably. For example, lithium-ion batteries have been commercially available since the early 1990’s, and have been rapidly decreasing in cost since. They come in a wide variety of sizes—they can power a cell phone or electric toothbrush, but they can also support utility-scale storage! In a recent lithium-ion utility pilot project in Sacramento, CA, six shipping-container sized batteries have been installed to produce 8MWh of electricity storage, about enough to power 800 homes for two hours. However, with a lifetime of 7 to 10 years, lithium-ion batteries have a shorter lifespan than other storage mediums that are in earlier stages of development. Which battery will be most effective for utility storage in Kern county will depend on how the technology develops in the coming few years, with a focus on optimizing systems to have long lifetimes as well as to be easy to maintain and replace, safe for routine use, suited for the desert heat, and cost-effective.
What are the societal and environmental considerations?
Generally, energy storage projects create few on-site jobs once constructed—most of the jobs that are created are software coders and maintenance employees. To keep these jobs local, there would need to be policy incentives or contract language that prioritizes local hiring. Battery facilities can be a community good, as they can serve to stabilize unreliability in the electrical grid – the benefits of this outside of industrial use within a carbon management park would depend on the scale of the battery storage installation.
There are no documented air pollution impacts of large-scale energy storage installations. The largest potential risk for battery storage installations is overheating, which can create a fire hazard. However, such a risk can be mitigated by compliance with state and federal policy and diligent site management.
In terms of the full life cycle of batteries, there is typically intensive mining to harness the necessary materials, as well as electronic waste at the end of the battery’s lifetime. There is a nascent industry in battery recycling that can partially mitigate both issues. Batteries must also be used to host energy that is very low-carbon or renewable in order to deliver climate benefits.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
What are the economic considerations?
In 2021, the estimated levelized cost of gridscale thermal and electric battery storage technologies ranged from about $0.10-1.30 USD/kWh. (For comparison, grid electricity in California is about $0.12-0.65 USD/kWh, and battery storage must be combined with the cost of energy production from wind or solar, about $0.03-0.05 USD/kWh.) Over time, the economics of large-scale battery storage projects are expected to improve drastically. California is already requiring utilities in the state to deploy more large-scale storage projects, encouraging economies of scale. The costs of these systems are also improving rapidly—as an example, the costs of lithium-ion batteries dropped by half between 2014 and 2018, a trend that is not uncommon among energy storage technologies. Between the policy mandates and favorable industry trends, battery storage projects are expected to be increasingly economically viable.

What is the technology and how does it work?
Water treatment technology is well-established, though processes differ based on the quality of intake water, the amount of water being processed, and the age of the facility. In general, water treatment relies on removing large objects first, then using chemicals and gravity to separate out small particulates in the water, and finishing with a disinfection step (typically using chlorine). Plants recycling wastewater will also produce byproducts, including biosolids and methane (natural gas).
Why would it be co-located with carbon management technology?
Kern County’s water supply is currently overallocated, with existing uses for agricultural, residential, and commercial purposes. To minimize impacts on the community and environment, it would be ideal to process and reuse as much water as possible onsite.
Many investigated industries, like green hydrogen from biomass, steel, and L-DAC, require water to operate, while some, like S-DAC and BiCRS, can actually generate water. If water can be cleaned and used directly onsite, the park could be largely self-sustaining in terms of water, depending on the mixture of industries developed.
What are the technical considerations?
Water treatment is an extremely energy-intensive process, using approximately 650 MWh per acre-foot of treated water—electricity costs often account for 25-40% of a facility’s operating costs. Ensuring there is enough renewable energy infrastructure in place to meet these high energy demands will be critical for ensuring climate benefits and economic resiliency for a treatment plant.
Furthermore, water treatment often takes up lots of space, with ground lakes or pools typically on-site to store influent water before treatment or effluent water before it goes out to customers. The Bakersfield Wastewater Treatment Plant No. 2, processing ~28,000 acre-feet of water per year, uses ~645 acres of land. Water can be moved to and from a facility using pipelines made of PVC or steel, depending on the necessary capacity and durability, with stainless steel being the more durable, but also more expensive, option.
What are the societal and environmental considerations?
Water should originate from or be recycled within a carbon management park as much as possible to avoid exacerbating land subsidence and other water shortage difficulties in Kern County.
Intentional design choices will need to be implemented to avoid light pollution (for safety reasons, water treatment facilities are required to be well-lit), disruptive noise from mechanical treatment operations, and if the facility processes wastewater, odor. Fortunately, innovative designs at existing facilities are already paving the way for less obstructive facilities in the future.
Water treatment can generate approximately a dozen jobs per plant, with most water treatment roles only requiring a high school diploma or GED accompanied with training for full certification. Water treatment roles are higher-paid than the national average, with hourly pay ranging from $15-40.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
What are the economic considerations?
The costs associated with water treatment vary considerably based on the quality of the influent (water entering the plant) and the necessary quality of effluent (water leaving the plant). Capital costs (the cost of building the facility) could range from half a million to tens of millions of dollars, depending on the required equipment, construction, and electricity costs. Furthermore, water treatment is often labor and energy-intensive, leading to high operating costs, while the price of water is kept artificially low in the market. As such, the profit incentives for private water treatment are negligible, thus choosing to treat and reuse water on-site depends on a facility’s industrial needs.

What is the technology and how does it work?
There are many processes available to make hydrogen, which are typically differentiated using a color system. Creating ‘green’ hydrogen through electrolysis, unlike other types of hydrogen production, does not generate carbon dioxide emissions—hence it is considered a clean energy industry, rather than a carbon management one. (Hydrogen production via gasification of biomass, which does generate carbon dioxide emissions that need to be captured and stored, is described as a carbon management industry. Other hydrogen production pathways - the other colors - are explained further in the downloadable report.)
Hydrogen production via electrolysis relies on a specialized device, an electrolyzer, to split water (H2O) into hydrogen and oxygen gas using a lot of energy and a catalyst, a material that facilitates the water splitting reaction. If this process is fueled by renewable energy like wind or solar, there are no emissions aside from the O2 gas, since the H2 is captured and compressed for sale. There are many types of electrolyzers – for a full explanation of the differences between alkaline, proton membrane exchange (PEM), and solid oxide electrolysis (SOE) systems, please see Section 6 of the full report.
Why would it be co-located with carbon management technology?
Renewable energy is good at meeting energy needs that are purely electrical, but not every industry’s needs are simple to electrify. If a process needs high heat energy, for example, energy straight from a wind turbine or solar field is poorly equipped to meet that need. Hydrogen can meet industrial heat needs for sectors like steel, BiCRS/BECCS or L-DAC, where this heat often originates currently from natural gas (methane).
A carbon management industrial park would also need a good amount of transportation infrastructure to move goods, such as feedstocks or co-products, and equipment. Hydrogen is being developed as a transportation fuel, potentially replacing diesel for long-haul road transport. Having an onsite supply of hydrogen fuel in a carbon management park would provide a low-carbon transportation solution for co-located industries.
What are the technical considerations?
Electrolysis is a water- and energy-intensive industry. For every kilogram of hydrogen gas produced, an electrolyzer consumes 12 liters of water, and requires 51-55 kWh of energy. To put that energy demand in perspective, if hydrogen were to replace US gasoline consumption (requiring about 135 million tons of hydrogen annually), it would use nearly twice the amount of electricity that currently powers the entire United States. Another consideration is that most electrolyzers need highly purified water to operate, thereby competing with other regional demands for clean, fresh water.
Working to minimize the burden of the water and energy use requirements will be helpful in scaling up hydrogen production via electrolysis. Currently, many experts are working on systems that can use wastewater or seawater for electrolysis to diminish the freshwater requirements, but lowering the energy requirements remains a challenge. Because water molecules are so strongly bonded, breaking them apart simply requires an extraordinary amount of energy.
What are the societal and environmental considerations?
Hydrogen production, transportation, and use are expected to create many jobs – as many as one million jobs nationwide could be tied to the hydrogen sector by 2030. However, these jobs will likely require training or trade certifications, so training pipelines should be set up in local communities to ensure jobs flow to local workers.
Hydrogen gas is very light and made of extremely small molecules, making it difficult to effectively store and transport. This can be a safety hazard, as hydrogen gas can be flammable and even explosive in air in high concentrations. Any hydrogen facility needs to be carefully designed and closely monitored in accordance with safety regulations, to prevent leaks and ensure the safety of on-site workers.
As mentioned in the technical considerations, electrolysis is very water-intensive. A newly developed green hydrogen production facility in western New York needs about 320,000 gallons of water for the 75,000 kg of hydrogen it produces each day. That translates to an annual use of 258-273 acre-feet per year, with the variation coming from cooling needs. For an arid region like Kern County, this could place undue strain on local water resources, especially in periods of drought. Using hydrogen for heating or transportation needs can also increase atmospheric water vapor, methane, and ozone through indirect chemical pathways, though it generates no carbon dioxide emissions. How significant these impacts are remains relatively unexplored, but given that such molecules all serve as short-term climate forcers when in the lower atmosphere, further research is warranted.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
What are the economic considerations?
Currently, hydrogen production via electrolysis is the most expensive production method for hydrogen, with solar-fueled electrolysis costing about $3 per kilogram of hydrogen produced in 2020. However, there are ways already available to reduce costs—since much of this cost is tied to energy use, when electrolysis can be done with excess renewable energy (ex. energy that would overload the grid and/or cause energy prices to go negative), the costs of electrolysis decline to $1.60/kilogram. Deploying electrolyzers is expected to get even cheaper with the deployment of multi-stack electrolyzer systems, which have a higher production capacity. Cost reductions of 20-40% are expected, increasing as the number of stacks increases.

What is the technology and how does it work?
CO2 utilization encompasses a whole range of potential industries, including fuels, food and beverages, building materials, concrete, and plastics and polymers.
The food and beverage industry can both directly use CO2 in applications like soft drink carbonation and dry ice, and use its chemically-converted products in alcoholic beverages. The other industries mentioned rely on converting CO2 through chemical or biological processes into other products, including lower-carbon fuels, building aggregates, lower-carbon concrete, and various plastics and foams. Processes which trap CO2 in long-lived materials, like building additives or concrete, can serve to remove CO2 from the atmosphere for years or decades, while industries like fuels and beverages will only remove CO2 for a short period. With these latter uses, the goal of CO2 utilization is to avoid the release of new carbon dioxide molecules through burning of fossil fuels or direct mining of CO2, by recycling carbon in and out of the atmosphere through technologies like direct air capture (DAC) or BiCRS.
Why would it be co-located with carbon management technology?
Although permanent underground storage will be necessary to meet California’s climate goals, use of CO2 can complement storage and reduce the carbon footprint of existing goods. By co-locating these industries with facilities that can capture carbon, they can share relevant infrastructure and reduce the costs of transporting CO2.
What are the technical considerations?
CO2-utilizing processes vary in their complexity, and even processes in the same industry can incorporate carbon in different ways. In the building materials industry, CO2 can be incorporated into cement mixtures, replacing the feedstock lime. Alternatively, concrete blocks or bricks need to be cured once made, which is traditionally done with water, but CO2 could be used to cure the blocks instead. CO2 also acts as a feedstock replacement in plastics manufacturing, although the process still requires virgin petrochemical materials to be successful. The largest expected use of CO2 in the future is for alternative fuels for transportation, but these fuels would release the CO2 into the atmosphere when the fuel is combusted.
What are the societal and environmental considerations?
CO2-utilizing industries could generate many jobs in the region, as plastics, building materials, and concrete manufacturing facilities are typically labor-intensive operations. In the U.S., nearly 100,000 people work in plastic manufacturing and another 12,500 in cement manufacturing – these jobs are largely blue-collar industrial jobs that do not require a college degree.
Although manufacturers integrating CO2 into their production process generally have a lower greenhouse gas emissions profile than their traditional competitors, additional considerations would need to be made regarding other pollutants common in these industries, and the water intensity of processing. In particular, cement manufacturing can release carbon monoxide, NOx, and other pollutants into the air. Petrochemical facilities release particulate matter, smog-creating volatile organic compounds (VOCs), and other pollutants. Water use can vary widely across industries; concrete water use typically declines with CO2 utilization, but water use would likely increase in sectors like chemical manufacturing. The severity and expected trends of water use would depend on the type of CO2-utilizing industry in question.
Regulatory Permitting: When sited in Kern County, California, all projects will be considered through a public process and environmental impacts will be reviewed and mitigated in accordance with the California Environmental Quality Act (CEQA).
What are the economic considerations?
Incorporating CO2 into the production process of existing products typically requires new equipment and facilities. In areas like plastics, the use of CO2 may reduce the per-unit cost of production, providing an avenue for recuperating higher upfront capital investments. However, for concrete and sustainable fuels, products incorporating CO2-utilization cost more than their traditional counterparts, and are likely to remain more expensive in the coming decades unless new mandates, regulations, or incentives alter the economic forecast. The federal 45Q tax credit does not account for uses other than enhanced oil recovery (EOR), which cannot use captured carbon under California’s SB 905 legislation. Generating alternative fuels using CO2 may qualify for credits under California’s Low Carbon Fuel Standard program, assuming these fuels are sold in-state for transportation use.
Furthermore, there may be existing policy restrictions that reduce the number of markets these goods can enter. For example, safety regulations on concrete for buildings, roads, and other materials have strict consistency guidelines; there would need to be further testing and regulatory updates to permit the sale of CO2-injected concrete materials to ensure they meet public safety standards.

